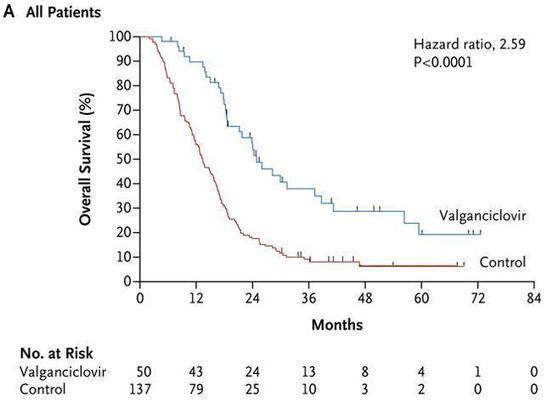
Your new post is loading...
Your new post is loading...

Emerging Therapies for Glioblastoma
Glioblastoma is an aggressive tumor with heterogeneous molecular features and complex host interactions, many of which are amenable to therapeutic intervention. Meaningful treatment advances will depend on identifying agents that target mechanistic vulnerabilities that are relevant to specific subgroups of patients; increasing patient enrollment into clinical trials is essential to accelerate the development of patient-tailored treatments.

AbbVie to Present Clinical Study Data on Potential New Oncology Medicines
Results from a Phase 1 study of ABT-414, an anti-EGFR (epidermal growth factor receptor) monoclonal antibody drug conjugate, in patients with newly diagnosed glioblastoma multiforme (GBM), the most common and most aggressive malignant primary brain tumor, will also be presented during an oral presentation.

Data being presented include results from a Phase 2 study evaluating the safety and efficacy of veliparib (ABT-888), a poly (adenosine diphosphate [ADP]–ribose) polymerase (PARP) inhibitor, in combination with two broad-acting chemotherapeutic medicines in patients with non-small cell lung cancer (NSCLC).
A new PARP Inhibitor
About ABT-767
ABT-767 is an investigational poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor being evaluated in multiple tumor types. PARP is a naturally-occurring enzyme in the body that repairs damage to DNA, and in certain types of cancers, repairs cancer cells. ABT-767 is currently being investigated in an ongoing Phase I clinical trial in patients with a variety of solid tumors. ABT-767 is an investigational compound and its efficacy and safety have not been established by the FDA.
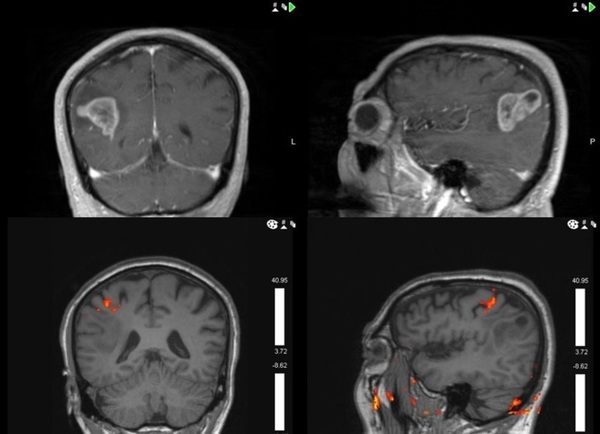
The Use of 5-ALA in Glioblastoma Resection: Two Cases with Long-Term Progression-Free Survival
In this paper, we report two cases of long-term progression-free survival in patients with glioblastoma undergoing FGR using of 5–aminolevulinic acid (5-ALA-FGR) at the University Hospitals-Case Medical Center.

Glioblastoma Multiforme Therapeutics in Asia-Pacific Markets to 2020
Novel Therapeutic Approaches Target High Unmet Need in Newly Diagnosed and Recurrent GBM: in-depth analysis of the Glioblastoma Multiforme (GBM) therapeutics market within the Asia-Pacific (APAC) region, covering Australia, China, India and Japan. The report provides estimates of market size for 2013, along with market forecasts until 2020. It also covers disease epidemiology, treatment algorithms, treatment patterns, and in-depth analysis of the marketed and pipeline products.

ImmunoCellular Therapeutics Receives Positive Regulatory Feedback on ICT-107 Supporting Advancement to Registrational Phase III Testing in Newly Diagnosed Glioblastoma (NYSE:IMUC)
During the second and third quarters of 2014, ImmunoCellular conducted discussions with regulatory authorities in the US and on the national level in Europe concerning the rationale, design and protocol of a phase III program for ICT-107 in newly diagnosed GBM.

Adenoviral vector-mediated gene therapy for gliomas: coming of age
DNAtrix Announces Treatment Of First Patient With DNX-2401 In Recurrent Glioblastoma Trial
DNAtrix, Inc., experts in oncolytic virus development, today announced that the first patient was treated with the company’s lead product, DNX-2401, a replication competent adenovirus plus gamma interferon in a randomized, multicenter, open-label Phase Ib study for patients with recurrent glioblastoma.

Asia-Pacific’s Glioblastoma Multiforme Treatment Market Value to More than Double by 2020
The Glioblastoma Multiforme (GBM) therapeutics market in Asia-Pacific (APAC) will more than double in value, from $49.4 million in 2013 to $105.8 million by 2020, at a Compound Annual Growth Rate (CAGR) of 11.5%, says a new report from business intelligence provider GBI Research.

Current role of anti-angiogenic strategies for glioblastoma
The authors aim to investigate the current role of anti-angiogenic strategies for glioblastoma. They suggest further research efforts are needed to determine optimal candidates for this treatment from a molecular standpoint, as well as to develop imaging tools capable of accurately identifying response and progression, and to establish new drug combinations that could result in unquestionable clinical benefit and improved survival in these patients.
Personalized brain vaccine for glioblastoma
CHICAGO (Ivanhoe Newswire) -- Glioblastoma is the most common and aggressive brain cancer in humans. The deadly cancer is usually treated with radiation and chemo, but the tumor inevitably comes back. Now a new treatment, using the patient’s tumor could help slow down its progression and help patients live longer.
New drug makes brain cancer cells explode
By screening over 1,000 different types of molecules, scientists have managed to identify a compound that can literally blow up tumor cells belonging to the most aggressive form of brain cancer — glioblastoma multiforme (GBM). The study, which was published today in Cell, was performed on mice, so the method will need a lot more testing before it's ready for human trials. But should the approach hold up, it could one day form the basis of an entirely new form of cancer treatment.

▶ Glioblastoma Multiforme - Symptoms, Diagnosis, Treatment
Gliomas are malignant tumors that start in the brain. Graded on a scale from 1-4, the most aggressive tumors are Grade 4 and are referred to as Glioblastoma Multiforme. In this video, Neurosurgeon Gail Rosseau, MD discusses the signs and symptoms of these tumors, as well as their diagnosis and treatment. For more information, visit http://www.northshore.org

Brain Tumor Treatment Breakthrough - Life Extension
Perhaps the most frightening malignancy is a brain cancer called glioblastoma multiforme. A recent study published in the New England Journal of Medicine may represent the most significant advance yet discovered in glioblastoma multiforme treatment.
Rapid growth forecast for glioblastoma multiforme therapeutics market
During the 2012-2019 forecast period, the GBM market is expected to grow rapidly from $305 million to $583 million. Due to the poor prognosis under currently available treatments, therapies with high potency are in strong demand in the GBM market.

Researchers identify new biomarker that predicts effectiveness of glioblastoma treatment
Researchers at the University of California, San Diego School of Medicine have identified a new biomarker that predicts whether glioblastoma – the most common form of primary brain cancer – will respond to chemotherapy. The findings are published in the July print issue of Oncotarget. “Every patient diagnosed with glioblastoma is treated with a chemotherapy called temozolomide. About 15 percent of these patients derive long-lasting benefit,” said Clark C. Chen, MD, PhD, vice-chairman of Academic Affairs, Division of Neurosurgery, UC San Diego School of Medicine and the study’s principal investigator.
Glioblastoma multiforme: Incurable brain cancer gene is silenced
Glioblastoma multiforme (GBM), the brain cancer that killed Sen. Edward Kennedy and kills approximately 13,000 Americans a year, is aggressive and incurable. Now a Northwestern University research team is the first to demonstrate delivery of a drug that turns off a critical gene in this complex cancer, ...